Effects and molecular mechanism of histone deacetylase 6 inhibitor Tubastatin A on the prolifera- tion and movement of human skin fibroblasts
-
摘要:
目的 探讨组蛋白脱乙酰酶6(HDAC6)抑制剂Tubastatin A对人皮肤成纤维细胞(HSF)增殖及运动性的影响及其可能的分子机制。 方法 采用实验研究方法。取对数生长期HSF,按随机数字表法分为阴性对照组及1 μmol/L Tubastatin A组、5 μmol/L Tubastatin A组、10 μmol/L Tubastatin A组。阴性对照组加入含终体积分数0.1%二甲基亚砜的DMEM培养液(以下简称完全培养液),其余3组分别加入含相应终物质的量浓度Tubastatin A的完全培养液。常规培养24 h后,采用细胞计数试剂盒8(CCK-8)法和5-乙炔基-2'-脱氧尿嘧啶核苷(EdU)染色检测细胞增殖活力;在活细胞工作站下观察细胞3 h内运动范围,计算细胞曲线运动速度;采用蛋白质印迹法检测胞外信号调节激酶1/2(ERK1/2)及磷酸化ERK1/2(p-ERK1/2)的蛋白表达量,并计算p-ERK1/2与ERK1/2比值,以此表示ERK1/2活性。CCK-8法行细胞增殖活力检测样本数为6,其余实验样本数为3。对数据行单因素方差分析及LSD检验。 结果 培养24 h后,CCK-8法和EdU染色显示,与阴性对照组比较,1 μmol/L Tubastatin A组、5 μmol/L Tubastatin A组、10 μmol/L Tubastatin A组细胞增殖活力均显著下降(P<0.01)。培养24 h后,CCK-8法显示,与1 μmol/L Tubastatin A组比较,10 μmol/L Tubastatin A组细胞增殖活力显著下降(P<0.05);EdU染色显示,与1 μmol/L Tubastatin A组比较,5 μmol/L Tubastatin A组、10 μmol/L Tubastatin A组细胞增殖活力显著下降(P<0.05或P<0.01)。观察3 h内,1 μmol/L Tubastatin A组、5 μmol/L Tubastatin A组、10 μmol/L Tubastatin A组细胞运动范围较阴性对照组明显缩小。观察3 h内,阴性对照组细胞曲线运动速度为(0.780±0.028)μm/min,明显快于1 μmol/L Tubastatin A组、5 μmol/L Tubastatin A组、10 μmol/L Tubastatin A组细胞的(0.594±0.023)、(0.469±0.028)、(0.391±0.021)μm/min(P<0.01);1 μmol/L Tubastatin A组细胞曲线运动速度明显快于5 μmol/L Tubastatin A组和10 μmol/L Tubastatin A组(P<0.01);5 μmol/L Tubastatin A组细胞曲线运动速度明显快于10 μmol/L Tubastatin A组(P<0.05)。培养24 h后,与阴性对照组比较,1 μmol/L Tubastatin A组、5 μmol/L Tubastatin A组、10 μmol/L Tubastatin A组细胞ERK1/2的活性显著下降(P<0.01);与1 μmol/L Tubastatin A组比,5 μmol/L Tubastatin A组和10 μmol/L Tubastatin A组细胞ERK1/2的活性显著下降(P<0.01);与5 μmol/L Tubastatin A组比较,10 μmol/L Tubastatin A组细胞ERK1/2的活性显著下降(P<0.05)。 结论 HDAC6抑制剂Tubastatin A可能通过抑制ERK1/2活性,从而抑制HSF增殖及运动。 -
关键词:
- 细胞增殖 /
- 细胞运动 /
- 组蛋白脱乙酰酶6 /
- Tubastatin A /
- 人皮肤成纤维细胞 /
- 胞外信号调节激酶1/2
Abstract:Objective To explore the effects and possible molecular mechanism of histone deacetylase 6 (HDAC6) inhibitor Tubastatin A on the proliferation and movement of human skin fibroblasts (HSFs). Methods The experimental research method was used. HSFs in logarithmic growth phase were taken and divided into negative control group, 1 μmol/L Tubastatin A group, 5 μmol/L Tubastatin A group, and 10 μmol/L Tubastatin A group according to the random number table. The HSFs in negative control group were added with Dulbecco′s modified eagle medium with the final volume fraction of 0.1% dimethyl sulfoxide (hereinafter referred to as the complete medium), and the other three groups were added with the complete medium with the corresponding final molarity of Tubastatin A. After 24 h of conventional culture, the cell proliferation activity was detected using cell counting kit 8 (CCK-8) method and 5-ethynyl-2'-deoxyuridine (EdU) staining; the range of motion of cells within 3 h was observed under the living cell workstation, and the curve movement velocity of the cells was calculated. The protein expressions of extracellular signal-regulated kinase 1/2 (ERK1/2) and phosphorylated ERK1/2 (p-ERK1/2) were detected by Western blotting, and the ratio of p-ERK1/2 to ERK1/2 was calculated to represent the activity of ERK1/2. The sample number in cell proliferation activity detection with CCK-8 method was 6, while the sample numbers in other experiments were 3. Data were statistically analyzed with one-way analysis of variance and least significant difference test. Results After 24 h of culture, CCK-8 method and EdU staining showed that compared with negative control group, the cell proliferation activities in 1 μmol/L Tubastatin A group, 5 μmol/L Tubastatin A group, and 10 μmol/L Tubastatin A group were significantly decreased (P<0.01). After 24 h of culture, CCK-8 method showed that compared with 1 μmol/L Tubastatin A group, the cell proliferation activity in 10 μmol/L Tubastatin A group was significantly decreased (P<0.05); EdU staining showed that compared with 1 μmol/L Tubastatin A group, the cell proliferation activities in 5 μmol/L Tubastatin A group and 10 μmol/L Tubastatin A group were significantly decreased (P<0.05 or P<0.01). Within 3 h of observation, the ranges of cell motion in 1 μmol/L Tubastatin A group, 5 μmol/L Tubastatin A group, and 10 μmol/L Tubastatin A group were obviously reduced compared with that in negative control group. Within 3 h of observation, the curve movement velocity of cells in negative control group was (0.780±0.028) μm/min, which was obviously faster than (0.594±0.023), (0.469±0.028), and (0.391±0.021) μm/min of 1 μmol/L Tubastatin A group, 5 μmol/L Tubastatin A group, and 10 μmol/L Tubastatin A group (P<0.01); the curve movement velocity of cells in 1 μmol/L Tubastatin A group was obviously faster than those in 5 μmol/L Tubastatin A group and 10 μmol/L Tubastatin A group (P<0.01); the curve movement velocity of cells in 5 μmol/L Tubastatin A group was obviously faster than that in 10 μmol/L Tubastatin A group (P<0.05). After 24 h of culture, compared with negative control group, the activities of ERK1/2 of cells in 1 μmol/L Tubastatin A group, 5 μmol/L Tubastatin A group, and 10 μmol/L Tubastatin A group were decreased significantly (P<0.01); compared with 1 μmol/L Tubastatin A group, the activities of ERK1/2 of cells in 5 μmol/L Tubastatin A group and 10 μmol/L Tubastatin A group were decreased significantly (P<0.01); compared with 5 μmol/L Tubastatin A group, the activity of ERK1/2 of cells in 10 μmol/L Tubastatin A group was decreased significantly (P<0.05). Conclusions HDAC6 inhibitor Tubastatin A may mediate the inhibitory effect on proliferation and movement of HSFs by inhibiting the activity of ERK1/2. -
参考文献
(35) [1] ZhuZ,HouQ,LiM,et al.Molecular mechanism of myofibroblast formation and strategies for clinical drugs treatments in hypertrophic scars[J].J Cell Physiol,2020,235(5):4109-4119.DOI: 10.1002/jcp.29302. [2] 马倩玉,武晓莉.增生性瘢痕和瘢痕疙瘩的最新治疗进展[J].组织工程与重建外科杂志,2020,16(1):1-5,26.DOI: 10.3969/j.issn.1673-0364.2020.01.001. [3] TanJ,WuJ.Current progress in understanding the molecular pathogenesis of burn scar contracture[J/OL].Burns Trauma,2017,5:14[2020-05-19]. https://pubmed.ncbi.nlm.nih.gov/28546987/. DOI: 10.1186/s41038-017-0080-1. [4] 刘杰,任淅,郭小伟,等.直流电场对BALB/c小鼠乳鼠真皮成纤维细胞定向迁移与排列的作用及其机制[J].中华烧伤杂志,2016,32(4):224-231.DOI: 10.3760/cma.j.issn.1009-2587.2016.04.007. [5] KunadisE,LakiotakiE,KorkolopoulouP,et al.Targeting post-translational histone modifying enzymes in glioblastoma[J].Pharmacol Ther,2021,220:107721.DOI: 10.1016/j.pharmthera.2020.107721. [6] ThakurA,TawaGJ,HendersonMJ,et al.Design, synthesis, and biological evaluation of quinazolin-4-one-based hydroxamic acids as dual PI3K/HDAC inhibitors[J].J Med Chem,2020,63(8):4256-4292.DOI: 10.1021/acs.jmedchem.0c00193. [7] MicelliC,RastelliG.Histone deacetylases: structural determinants of inhibitor selectivity[J].Drug Discov Today,2015,20(6):718-735.DOI: 10.1016/j.drudis.2015.01.007. [8] EckerJ,ThatikondaV,SigismondoG,et al.Reduced chromatin binding of MYC is a key effect of HDAC inhibition in MYC amplified medulloblastoma[J].Neuro Oncol,2021,23(2):226-239.DOI: 10.1093/neuonc/noaa191. [9] 张文斌,王瑶,焦方舟.组蛋白去乙酰化酶6在相关疾病中的研究进展[J].疑难病杂志,2019,18(10):1062-1066.DOI: 10.3969/j.issn.1671-6450.2019.10.023. [10] ZhangL,DengM,LuA,et al.Sodium butyrate attenuates angiotensin II-induced cardiac hypertrophy by inhibiting COX2/PGE2 pathway via a HDAC5/HDAC6-dependent mechanism[J].J Cell Mol Med,2019,23(12):8139-8150.DOI: 10.1111/jcmm.14684. [11] LiangT,FangH.Structure, functions and selective inhibitors of HDAC6[J].Curr Top Med Chem,2018,18(28):2429-2447.DOI: 10.2174/1568026619666181129141822. [12] ZhangJ,LiL,ZhangQ,et al.Microtubule-associated protein 4 phosphorylation regulates epidermal keratinocyte migration and proliferation[J].Int J Biol Sci,2019,15(9):1962-1976.DOI: 10.7150/ijbs.35440. [13] ZhangJ,ZhangC,JiangX,et al.Involvement of autophagy in hypoxia-BNIP3 signaling to promote epidermal keratinocyte migration[J].Cell Death Dis,2019,10(3):234.DOI: 10.1038/s41419-019-1473-9. [14] LiuSS,WuF,JinYM,et al.HDAC11: a rising star in epigenetics[J].Biomed Pharmacother,2020,131:110607.DOI: 10.1016/j.biopha.2020.110607. [15] MagupalliVG,NegroR,TianY,et al.HDAC6 mediates an aggresome-like mechanism for NLRP3 and pyrin inflammasome activation[J].Science,2020,369(6510):eaas8995. DOI: 10.1126/science.aas8995. [16] PulyaS,AminSA,AdhikariN,et al.HDAC6 as privileged target in drug discovery: a perspective[J].Pharmacol Res,2021,163:105274.DOI: 10.1016/j.phrs.2020.105274. [17] MonavarianM,KaderS,MoeinzadehS,et al.Regenerative scar-free skin wound healing[J].Tissue Eng Part B Rev,2019,25(4):294-311.DOI: 10.1089/ten.TEB.2018.0350. [18] BhattacharyyaS,WangW,QinW,et al.TLR4-dependent fibroblast activation drives persistent organ fibrosis in skin and lung[J].JCI Insight,2018,3(13):e98850. DOI: 10.1172/jci.insight.98850. [19] PangM,ZhuangS.Histone deacetylase: a potential therapeutic target for fibrotic disorders[J].J Pharmacol Exp Ther,2010,335(2):266-272.DOI: 10.1124/jpet.110.168385. [20] ChenPJ,HuangC,MengXM,et al.Epigenetic modifications by histone deacetylases: biological implications and therapeutic potential in liver fibrosis[J].Biochimie,2015,116:61-69.DOI: 10.1016/j.biochi.2015.06.016. [21] TaoH,ShiKH,YangJJ,et al.Histone deacetylases in cardiac fibrosis: current perspectives for therapy[J].Cell Signal,2014,26(3):521-527.DOI: 10.1016/j.cellsig.2013.11.037. [22] DuW,WangN,LiF,et al.STAT3 phosphorylation mediates high glucose-impaired cell autophagy in an HDAC1-dependent and -independent manner in Schwann cells of diabetic peripheral neuropathy[J].FASEB J,2019,33(7):8008-8021.DOI: 10.1096/fj.201900127R. [23] MohantyA,SandovalN,PhanA,et al.Regulation of SOX11 expression through CCND1 and STAT3 in mantle cell lymphoma[J].Blood,2019,133(4):306-318.DOI: 10.1182/blood-2018-05-851667. [24] XuZ,JiaK,WangH,et al.METTL14-regulated PI3K/Akt signaling pathway via PTEN affects HDAC5-mediated epithelial-mesenchymal transition of renal tubular cells in diabetic kidney disease[J].Cell Death Dis,2021,12(1):32.DOI: 10.1038/s41419-020-03312-0. [25] El AyadiA,JayJW,PrasaiA.Current approaches targeting the wound healing phases to attenuate fibrosis and scarring[J].Int J Mol Sci,2020,21(3):1105. DOI: 10.3390/ijms21031105. [26] SharmaA,MehanMM,SinhaS,et al.Trichostatin a inhibits corneal haze in vitro and in vivo[J].Invest Ophthalmol Vis Sci,2009,50(6):2695-2701.DOI: 10.1167/iovs.08-2919. [27] ChoiYJ,KangMH,HongK,et al.Tubastatin A inhibits HDAC and Sirtuin activity rather than being a HDAC6-specific inhibitor in mouse oocytes[J].Aging (Albany NY),2019,11(6):1759-1777.DOI: 10.18632/aging.101867. [28] WangZ,HuP,TangF,et al.HDAC6 promotes cell proliferation and confers resistance to temozolomide in glioblastoma[J].Cancer Lett,2016,379(1):134-142.DOI: 10.1016/j.canlet.2016.06.001. [29] DeskinB,YinQ,ZhuangY,et al.Inhibition of HDAC6 attenuates tumor growth of non-small cell lung cancer[J].Transl Oncol,2020,13(2):135-145.DOI: 10.1016/j.tranon.2019.11.001. [30] PapaS,ChoyPM,BubiciC.The ERK and JNK pathways in the regulation of metabolic reprogramming[J].Oncogene,2019,38(13):2223-2240.DOI: 10.1038/s41388-018-0582-8. [31] AokiK,KondoY,NaokiH,et al.Propagating wave of ERK activation orients collective cell migration[J].Dev Cell,2017,43(3):305-317.e5.DOI: 10.1016/j.devcel.2017.10.016. [32] DangCV,KimJW,GaoP,et al.The interplay between MYC and HIF in cancer[J].Nat Rev Cancer,2008,8(1):51-56.DOI: 10.1038/nrc2274. [33] KimJ,KimB,KimSM,et al.Hypoxia-induced epithelial-to-mesenchymal transition mediates fibroblast abnormalities via ERK activation in cutaneous wound healing[J].Int J Mol Sci,2019,20(10):2546. DOI: 10.3390/ijms20102546. [34] WangW,QuM,XuL,et al.Sorafenib exerts an anti-keloid activity by antagonizing TGF-β/Smad and MAPK/ERK signaling pathways[J].J Mol Med (Berl),2016,94(10):1181-1194.DOI: 10.1007/s00109-016-1430-3. [35] ZhangSL, ZhuHY, ZhouBY, et al. Histone deacetylase 6 is overexpressed and promotes tumor growth of colon cancer through regulation of the MAPK/ERK signal pathway[J]. Onco Targets Ther, 2019, 12: 2409-2419. DOI: 10.2147/OTT.S194986. -
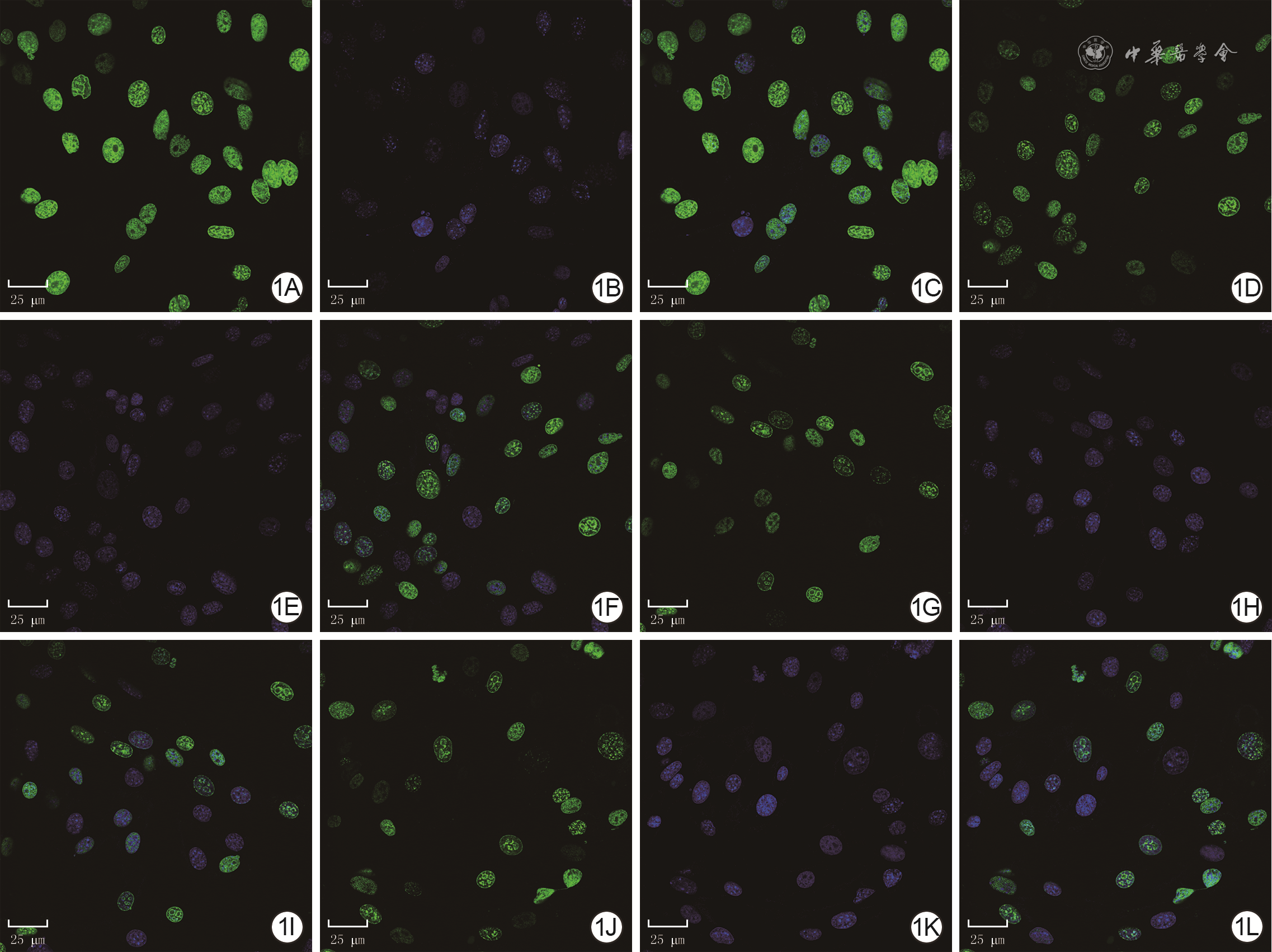
1 EdU染色法检测阴性对照组及Tubastatin A处理3组HSF增殖活力 EdU-DAPI×630,图中标尺为25 μm。1A、1B、1C.分别为阴性对照组细胞EdU染色、细胞核染色、细胞EdU染色与细胞核染色重叠图片,细胞核完整,EdU阳性染色细胞较多;1D、1E、1F.分别为1 μmol/L Tubastatin A组细胞EdU染色、细胞核染色、细胞EdU染色与细胞核染色重叠图片,细胞核完整,EdU阳性染色细胞较阴性对照组明显减少;1G、1H、1I及1J、1K、1L.分别为5 μmol/L Tubastatin A组、10 μmol/L Tubastatin A组细胞EdU染色、细胞核染色、细胞EdU染色与细胞核染色重叠图片,EdU阳性染色细胞均较1 μmol/L Tubastatin A组减少
注:HSF为人皮肤成纤维细胞,EdU为5-乙炔基-2'-脱氧尿嘧啶核苷,DAPI为4',6-二脒基-2-苯基吲哚;细胞EdU阳性染色为绿色,细胞核阳性染色为蓝色,绿色+蓝色双荧光染色为增殖的HSF
-








 下载:
下载: