National expert consensus on the diagnosis and surgical treatment of diabetic foot ulcers complicated with lower extremity vasculopathy (2024 version)
-
摘要: 糖尿病足溃疡合并下肢血管病变具有发病率高、愈合缓慢、预后差的特点,如不经规范治疗易导致截肢甚至危及生命。针对合并下肢血管病变的治疗对改善糖尿病足溃疡的愈合进程至关重要,在临床实践中逐渐得到重视。近年来,关于糖尿病足溃疡合并下肢血管病变的临床研究已见诸多报道,为了进一步规范临床诊疗,由中国老年医学学会烧创伤分会、中华医学会烧伤外科学分会、中国医师协会创面修复专业委员会牵头的专家组共同审议并编写了《糖尿病足溃疡合并下肢血管病变的外科诊疗全国专家共识(2024版)》。该共识以文献证据为基础,内容涵盖了糖尿病足溃疡合并下肢血管病变的疾病特征、临床诊疗循证证据以及新技术和新治疗方法的应用,旨在为临床工作者提供关于糖尿病足溃疡合并下肢血管病变最佳筛查和诊疗方法的明确指导,希望为从事糖尿病足创面治疗的医务人员提供规范性的临床实践依据。Abstract: Diabetic foot ulcers complicated with lower extremity vasculopathy possess the characteristics of high incidence, slow healing, and poor prognosis, which may eventually lead to amputation or even life-threatening if not treated properly. The treatment of complicated lower extremity vasculopathy is vital to improve the healing process of diabetic foot ulcers, which has gradually received attention in clinical practice. Recently, a number of clinical trials on diabetic foot ulcers complicated with lower extremity vasculopathy were reported. In order to further standardize the clinical diagnosis and treatment of diabetic foot ulcers complicated with lower extremity vasculopathy, an expert group headed by Burns and Trauma Branch of Chinese Geriatrics Society, Chinese Burn Association, and Wound Repair Professional Committee of Chinese Medical Doctor Association deliberated and compiled the National expert consensus on the diagnosis and surgical treatment of diabetic foot ulcers complicated with lower extremity vasculopathy (2024 version) together. This consensus is based on evidences from the literature, covers the disease characteristics, evidence-based evidence of clinical diagnosis and treatment, as well as the application of new technologies and new treatment approaches of diabetic foot ulcers complicated with lower extremity vasculopathy. The goal of this consensus is to provide clear guidance to practitioners on the best approaches for screening, diagnosing, and treating diabetic foot ulcers complicated with lower extremity vasculopathy in individuals, hoping to provide a normative clinical practice basis for medical staff engaged in the treatment of diabetic foot wounds.
-
Key words:
- Diabetic foot /
- Diabetic angiopathies /
- Peripheral arterial disease /
- Microcirculation /
- Amputation /
- Wound repair /
- Expert consensus
-
参考文献
(84) [1] ArmstrongDG, BoultonAJM, BusSA. Diabetic foot ulcers and their recurrence[J]. N Engl J Med, 2017, 376(24): 2367-2375. DOI: 10.1056/NEJMra1615439. [2] ZhangYQ, LazzariniPA, McphailSM, et al. Global disability burdens of diabetes-related lower-extremity complications in 1990 and 2016[J]. Diabetes Care, 2020, 43(5): 964-974. DOI: 10.2337/dc19-1614. [3] MorbachS,FurchertH,GröblinghoffU,et al.Long-term prognosis of diabetic foot patients and their limbs: amputation and death over the course of a decade[J].Diabetes Care,2012,35(10):2021-2027.DOI: 10.2337/dc12-0200. [4] WagnerFWJr.The dysvascular foot: a system for diagnosis and treatment[J].Foot Ankle,1981,2(2):64-122.DOI: 10.1177/107110078100200202. [5] LipskyBA,SennevilleÉ,AbbasZG,et al.Guidelines on the diagnosis and treatment of foot infection in persons with diabetes (IWGDF 2019 update)[J].Diabetes Metab Res Rev,2020,36Suppl 1:Se3280.DOI: 10.1002/dmrr.3280. [6] LaveryLA,ArmstrongDG,HarklessLB.Classification of diabetic foot wounds[J].J Foot Ankle Surg,1996,35(6):528-531.DOI: 10.1016/s1067-2516(96)80125-6. [7] InceP,AbbasZG,LutaleJK,et al.Use of the SINBAD classification system and score in comparing outcome of foot ulcer management on three continents[J].Diabetes Care,2008,31(5):964-967.DOI: 10.2337/dc07-2367. [8] SrMills JL, ConteMS, ArmstrongDG,et al.The Society for Vascular Surgery Lower Extremity Threatened Limb Classification System: risk stratification based on wound, ischemia, and foot infection (WIfI)[J].J Vasc Surg,2014,59(1):220-234.e1-2.DOI: 10.1016/j.jvs.2013.08.003. [9] NorgrenL, HiattWR, DormandyJA, et al. Inter-society consensus for the management of peripheral arterial disease (TASC Ⅱ)[J]. J Vasc Surg, 2007, 45 Suppl S: S5-67. DOI: 10.1016/j.jvs.2006.12.037. [10] GBD 2021 Diabetes Collaborators.Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: a systematic analysis for the Global Burden of Disease Study 2021[J].Lancet,2023,402(10397):203-234.DOI: 10.1016/S0140-6736(23)01301-6. [11] 管珩,刘志民,李光伟,等.50岁以上糖尿病人群周围动脉闭塞性疾病相关因素分析[J].中华医学杂志,2007,87(1):23-27.DOI: 10.3760/j:issn:0376-2491.2007.01.008. [12] UçkayI,GarianiK,PatakyZ,et al.Diabetic foot infections: state-of-the-art[J].Diabetes Obes Metab,2014,16(4):305-316.DOI: 10.1111/dom.12190. [13] BianchettiG, RizzoGE, SerantoniC, et al. Spatial reorganization of liquid crystalline domains of red blood cells in type 2 diabetic patients with peripheral artery disease[J]. Int J Mol Sci, 2022,23(19):11126. DOI: 10.3390/ijms231911126. [14] LangeS,DiehmC,DariusH,et al.High prevalence of peripheral arterial disease and low treatment rates in elderly primary care patients with diabetes[J].Exp Clin Endocrinol Diabetes,2004,112(10):566-573.DOI: 10.1055/s-2004-830408. [15] NickinsonATO, ColesB, ZaccardiF, et al. Missed opportunities for timely recognition of chronic limb threatening ischaemia in patients undergoing a major amputation: a population based cohort study using the UK's Clinical Practice Research Datalink[J]. Eur J Vasc Endovasc Surg, 2020, 60(5): 703-710. DOI: 10.1016/j.ejvs.2020.05.010. [16] AndrosG.Diagnostic and therapeutic arterial interventions in the ulcerated diabetic foot[J].Diabetes Metab Res Rev,2004,20 Suppl 1:S29-33.DOI: 10.1002/dmrr.468. [17] BoykoEJ.How to use clinical signs and symptoms to estimate the probability of limb ischaemia in patients with a diabetic foot ulcer[J].Diabetes Metab Res Rev,2020,36Suppl 1:Se3241.DOI: 10.1002/dmrr.3241. [18] GershaterMA,LöndahlM,NybergP,et al.Complexity of factors related to outcome of neuropathic and neuroischaemic/ischaemic diabetic foot ulcers: a cohort study[J].Diabetologia,2009,52(3):398-407.DOI: 10.1007/s00125-008-1226-2. [19] ReardonR,SimringD,KimB,et al.The diabetic foot ulcer[J].Aust J Gen Pract,2020,49(5):250-255.DOI: 10.31128/AJGP-11-19-5161. [20] LonderoLS,LindholtJS,ThomsenMD,et al.Pulse palpation is an effective method for population-based screening to exclude peripheral arterial disease[J].J Vasc Surg,2016,63(5):1305-1310.DOI: 10.1016/j.jvs.2015.11.044. [21] American Diabetes Association. 10. Microvascular complications and foot care: Standards of Medical Care in Diabetes-2018[J]. Diabetes Care, 2018, 41 (Suppl 1): S105-118. DOI: 10.2337/dc18-S010. [22] ArmstrongDW,TobinC,MatangiMF.The accuracy of the physical examination for the detection of lower extremity peripheral arterial disease[J].Can J Cardiol,2010,26(10):e346-350.DOI: 10.1016/s0828-282x(10)70467-0. [23] PolonskyTS, McdermottMM. Lower extremity peripheral artery disease without chronic limb-threatening ischemia: a review[J]. JAMA, 2021, 325(21): 2188-2198. DOI: 10.1001/jama.2021.2126. [24] XuDC,LiJ,ZouLL,et al.Sensitivity and specificity of the ankle--brachial index to diagnose peripheral artery disease: a structured review[J].Vasc Med,2010,15(5):361-369.DOI: 10.1177/1358863X10378376. [25] HirschAT,HaskalZJ,HertzerNR,et al.ACC/AHA 2005 guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): executive summary a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease) endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation[J].J Am Coll Cardiol,2006,47(6):1239-1312.DOI: 10.1016/j.jacc.2005.10.009. [26] Gerhard-HermanMD, GornikHL, BarrettC, et al. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines[J]. Circulation, 2017, 135(12): e726-e779. DOI: 10.1161/CIR.0000000000000471. [27] ForsytheRO, ApelqvistJ, BoykoEJ, et al. Effectiveness of bedside investigations to diagnose peripheral artery disease among people with diabetes mellitus: a systematic review[J]. Diabetes Metab Res Rev, 2020, 36Suppl 1:Se3277. DOI: 10.1002/dmrr.3277. [28] HøyerC, StrandbergJ, Overvad JordansenMK, et al. The ability of the toe-brachial index to predict the outcome of treadmill exercise testing in patients with a normal resting ankle-brachial index[J]. Ann Vasc Surg, 2020, 64: 263-269. DOI: 10.1016/j.avsg.2019.10.041. [29] LeenstraB, de KleijnR, KuppensG, et al. Photo-optical transcutaneous oxygen tension measurement is of added value to predict diabetic foot ulcer healing: an observational study[J]. J Clin Med, 2020, 9(10):3291. DOI: 10.3390/jcm9103291. [30] American Diabetes Association. 2. Classification and diagnosis of diabetes: Standards of Medical Care in Diabetes-2020[J]. Diabetes Care, 2020, 43 (Suppl 1): S14-31. DOI: 10.2337/dc20-S002. [31] PieruzziL, NapoliV, GorettiC, et al. Ultrasound in the modern management of the diabetic foot syndrome: a multipurpose versatile toolkit[J]. Int J Low Extrem Wounds, 2020, 19(4): 315-333. DOI: 10.1177/1534734620948351. [32] CollinsR,BurchJ,CrannyG,et al.Duplex ultrasonography, magnetic resonance angiography, and computed tomography angiography for diagnosis and assessment of symptomatic, lower limb peripheral arterial disease: systematic review[J].BMJ,2007,334(7606):1257.DOI: 10.1136/bmj.39217.473275.55. [33] LiangHL. Doppler flow measurement of lower extremity arteries adjusted by pulsatility index[J]. AJR Am J Roentgenol, 2020, 214(1): 10-17. DOI: 10.2214/AJR.19.21280. [34] OferA,NiteckiSS,LinnS,et al.Multidetector CT angiography of peripheral vascular disease: a prospective comparison with intraarterial digital subtraction angiography[J].AJR Am J Roentgenol,2003,180(3):719-724.DOI: 10.2214/ajr.180.3.1800719. [35] MenkeJ,LarsenJ.Meta-analysis: accuracy of contrast-enhanced magnetic resonance angiography for assessing steno-occlusions in peripheral arterial disease[J].Ann Intern Med,2010,153(5):325-334.DOI: 10.7326/0003-4819-153-5-201009070-00007. [36] Varga-SzemesA,PenmetsaM,EmrichT,et al.Diagnostic accuracy of non-contrast quiescent-interval slice-selective (QISS) MRA combined with MRI-based vascular calcification visualization for the assessment of arterial stenosis in patients with lower extremity peripheral artery disease[J].Eur Radiol,2021,31(5):2778-2787.DOI: 10.1007/s00330-020-07386-4. [37] HentschA,AschauerMA,BalzerJO,et al.Gadobutrol-enhanced moving-table magnetic resonance angiography in patients with peripheral vascular disease: a prospective, multi-centre blinded comparison with digital subtraction angiography[J].Eur Radiol,2003,13(9):2103-2114.DOI: 10.1007/s00330-003-1844-5. [38] JeonBJ,ChoiHJ,KangJS,et al.Comparison of five systems of classification of diabetic foot ulcers and predictive factors for amputation[J].Int Wound J,2017,14(3):537-545.DOI: 10.1111/iwj.12642. [39] Monteiro-SoaresM,RussellD,BoykoEJ,et al.Guidelines on the classification of diabetic foot ulcers (IWGDF 2019)[J].Diabetes Metab Res Rev,2020,36Suppl 1:Se3273.DOI: 10.1002/dmrr.3273. [40] DuttaA, BhansaliA, RastogiA. Early and intensive glycemic control for diabetic foot ulcer healing: a prospective observational nested cohort study[J]. Int J Low Extrem Wounds, 2023,22(3):578-587. DOI: 10.1177/15347346211033458. [41] LinX,ChenYY,LuW,et al.Ultrasonography evaluation on the protective effect of combination therapy of beraprost sodium and aspirin on arteries occlusion and stiffness in patients with type 2 diabetes mellitus - a prospective, randomized study[J].BMC Endocr Disord,2022,22(1):87.DOI: 10.1186/s12902-022-01007-5. [42] DeedwaniaP.Hypertension, dyslipidemia, and insulin resistance in patients with diabetes mellitus or the cardiometabolic syndrome: benefits of vasodilating β-blockers[J].J Clin Hypertens (Greenwich),2011,13(1):52-59.DOI: 10.1111/j.1751-7176.2010.00386.x. [43] SagarRC,NaseemKM,AjjanRA.Antiplatelet therapies in diabetes[J].Diabet Med,2020,37(5):726-734.DOI: 10.1111/dme.14291. [44] AjjanRA,KietsirirojeN,BadimonL,et al.Antithrombotic therapy in diabetes: which, when, and for how long?[J].Eur Heart J,2021,42(23):2235-2259.DOI: 10.1093/eurheartj/ehab128. [45] GrazianiL,SilvestroA,BertoneV,et al.Vascular involvement in diabetic subjects with ischemic foot ulcer: a new morphologic categorization of disease severity[J].Eur J Vasc Endovasc Surg,2007,33(4):453-460.DOI: 10.1016/j.ejvs.2006.11.022. [46] HinchliffeRJ,BrownriggJRW,AndrosG,et al.Effectiveness of revascularization of the ulcerated foot in patients with diabetes and peripheral artery disease: a systematic review[J].Diabetes Metab Res Rev,2016,32 Suppl 1:S136-144.DOI: 10.1002/dmrr.2705. [47] HoulindK,ChristensenJK,JepsenJM.Vein arterialization for lower limb revascularization[J].J Cardiovasc Surg (Torino),2016,57(2):266-272. [48] GuptaPK,ShivashankarP,RajkumarM,et al.Label extension, single-arm, phase Ⅲ study shows efficacy and safety of stempeucel ® in patients with critical limb ischemia due to atherosclerotic peripheral arterial disease[J].Stem Cell Res Ther,2023,14(1):60.DOI: 10.1186/s13287-023-03292-w. [49] BhatMA,ZarooMI,DarziMA.Omental transplantation for critical limb ischemia in Buerger's disease[J].Plast Reconstr Surg,2007,119(6):1979-1980.DOI: 10.1097/01.prs.0000259775.68749.4a. [50] MarsicoG, Martin-SaldañaS, PanditA. Therapeutic biomaterial approaches to alleviate chronic limb threatening ischemia[J]. Adv Sci (Weinh), 2021, 8(7): 2003119. DOI: 10.1002/advs.202003119. [51] FagliaE,Dalla PaolaL,ClericiG,et al.Peripheral angioplasty as the first-choice revascularization procedure in diabetic patients with critical limb ischemia: prospective study of 993 consecutive patients hospitalized and followed between 1999 and 2003[J].Eur J Vasc Endovasc Surg,2005,29(6):620-627.DOI: 10.1016/j.ejvs.2005.02.035. [52] SrMills JL. Open bypass and endoluminal therapy: complementary techniques for revascularization in diabetic patients with critical limb ischaemia[J].Diabetes Metab Res Rev,2008,24 Suppl 1:S34-39.DOI: 10.1002/dmrr.829. [53] BredahlK,JensenLP,SchroederTV,et al.Mortality and complications after aortic bifurcated bypass procedures for chronic aortoiliac occlusive disease[J].J Vasc Surg,2015,62(1):75-82.DOI: 10.1016/j.jvs.2015.02.025. [54] HoVT,GologorskyR,KibrikP,et al.Open, percutaneous, and hybrid deep venous arterialization technique for no-option foot salvage[J].J Vasc Surg,2020,71(6):2152-2160.DOI: 10.1016/j.jvs.2019.10.085. [55] BrownriggJRW,HinchliffeRJ,ApelqvistJ,et al.Performance of prognostic markers in the prediction of wound healing or amputation among patients with foot ulcers in diabetes: a systematic review[J].Diabetes Metab Res Rev,2016,32 Suppl 1:S128-135.DOI: 10.1002/dmrr.2704. [56] HinchliffeRJ,BrownriggJRW,ApelqvistJ,et al.IWGDF guidance on the diagnosis, prognosis and management of peripheral artery disease in patients with foot ulcers in diabetes[J].Diabetes Metab Res Rev,2016,32 Suppl 1:S37-44.DOI: 10.1002/dmrr.2698. [57] CaselliA,LatiniV,LapennaA,et al.Transcutaneous oxygen tension monitoring after successful revascularization in diabetic patients with ischaemic foot ulcers[J].Diabet Med,2005,22(4):460-465.DOI: 10.1111/j.1464-5491.2005.01446.x. [58] ZubairM,AhmadJ.Transcutaneous oxygen pressure (TcPO 2) and ulcer outcome in diabetic patients: is there any correlation?[J].Diabetes Metab Syndr,2019,13(2):953-958.DOI: 10.1016/j.dsx.2018.12.008. [59] ShahP, InturiR, AnneD, et al. Wagner's classification as a tool for treating diabetic foot ulcers: our observations at a suburban teaching hospital[J]. Cureus, 2022, 14(1): e21501. DOI: 10.7759/cureus.21501. [60] LiMQ.Guidelines and standards for comprehensive clinical diagnosis and interventional treatment for diabetic foot in China (issue 7.0)[J].J Interv Med,2021,4(3):117-129.DOI: 10.1016/j.jimed.2021.07.003. [61] 谢闪亮,郭光华,闵定宏.封闭负压引流技术在创面愈合中的应用及机制研究进展[J].中华烧伤杂志,2017,33(6):397-400.DOI: 10.3760/cma.j.issn.1009-2587.2017.06.024. [62] BlumePA,WaltersJ,PayneW,et al.Comparison of negative pressure wound therapy using vacuum-assisted closure with advanced moist wound therapy in the treatment of diabetic foot ulcers: a multicenter randomized controlled trial[J].Diabetes Care,2008,31(4):631-636.DOI: 10.2337/dc07-2196. [63] SaxenaV,HwangCW,HuangS,et al.Vacuum-assisted closure: microdeformations of wounds and cell proliferation[J].Plast Reconstr Surg,2004,114(5):1086-1096; discussion 1097-1098.DOI: 10.1097/01.prs.0000135330.51408.97. [64] JiSZ,LiuXB,HuangJ,et al.Consensus on the application of negative pressure wound therapy of diabetic foot wounds[J/OL].Burns Trauma,2021,9:tkab018[2023-11-22]. https://pubmed.ncbi.nlm.nih.gov/34212064/.DOI: 10.1093/burnst/tkab018. [65] GreeneAK,PuderM,RoyR,et al.Microdeformational wound therapy: effects on angiogenesis and matrix metalloproteinases in chronic wounds of 3 debilitated patients[J].Ann Plast Surg,2006,56(4):418-422.DOI: 10.1097/01.sap.0000202831.43294.02. [66] RupertP.Human acellular dermal wound matrix for complex diabetic wounds[J].J Wound Care,2016,25(4):S17-18, S20-21.DOI: 10.12968/jowc.2016.25.Sup4.S17. [67] Mendame EhyaRE, ZhangH, QiB, et al. Application and clinical effectiveness of antibiotic-loaded bone cement to promote soft tissue granulation in the treatment of neuropathic diabetic foot ulcers complicated by osteomyelitis: a randomized controlled trial[J]. J Diabetes Res, 2021, 2021: 9911072. DOI: 10.1155/2021/9911072. [68] LiuX, LiangJL, ZaoJ, et al. Vacuum sealing drainage treatment combined with antibiotic-impregnated bone cement for treatment of soft tissue defects and infection[J]. Med Sci Monit, 2016, 22: 1959-1965. DOI: 10.12659/msm.896108. [69] JiangX,XuY,JiaoGQ,et al.The combined application of antibiotic-loaded bone cement and vacuum sealing drainage for sternal reconstruction in the treatment of deep sternal wound infection[J].J Cardiothorac Surg,2022,17(1):209.DOI: 10.1186/s13019-022-01951-2. [70] IlizarovGA.Clinical application of the tension-stress effect for limb lengthening[J].Clin Orthop Relat Res,1990(250):8-26. [71] ZuoQ,GaoF,SongHH,et al.Application of Ilizarov transverse tibial bone transport and microcirculation reconstruction in the treatment of chronic ischemic diseases in lower limbs[J].Exp Ther Med,2018,16(2):1355-1359.DOI: 10.3892/etm.2018.6321. [72] OuSJ, XuCP, YangY, et al. Transverse tibial bone transport enhances distraction osteogenesis and vascularization in the treatment of diabetic foot[J]. Orthop Surg, 2022, 14(9): 2170-2179. DOI: 10.1111/os.13416. [73] 高磊,王硕,王雷,等.皮肤牵张闭合器在糖尿病足创面修复中的应用[J].中国修复重建外科杂志,2018,32(5):591-595.DOI: 10.7507/1002-1892.201801104. [74] 计鹏,张月,胡大海,等.皮肤牵张器联合负压封闭引流修复糖尿病足创面的临床效果[J].中华烧伤杂志,2020,36(11):1035-1039.DOI: 10.3760/cma.j.cn501120-20200621-00318. [75] DriverVR,LaveryLA,ReyzelmanAM,et al.A clinical trial of Integra Template for diabetic foot ulcer treatment[J].Wound Repair Regen,2015,23(6):891-900.DOI: 10.1111/wrr.12357. [76] LiangYP, HeJH, GuoBL. Functional hydrogels as wound dressing to enhance wound healing[J]. ACS Nano, 2021, 15(8): 12687-12722. DOI: 10.1021/acsnano.1c04206. [77] MasriS, ZawaniM, ZulkifleeI, et al. Cellular interaction of human skin cells towards natural bioink via 3D-bioprinting technologies for chronic wound: a comprehensive review[J]. Int J Mol Sci, 2022, 23(1):476. DOI: 10.3390/ijms23010476. [78] YammineK, AssiC. A meta-analysis of the outcomes of split-thickness skin graft on diabetic leg and foot ulcers[J]. Int J Low Extrem Wounds, 2019, 18(1): 23-30. DOI: 10.1177/1534734619832123. [79] Fitzgerald O'ConnorEJ, VeselyM, HoltPJ,et al.A systematic review of free tissue transfer in the management of non-traumatic lower extremity wounds in patients with diabetes[J].Eur J Vasc Endovasc Surg,2011,41(3):391-399.DOI: 10.1016/j.ejvs.2010.11.013. [80] OhTS,LeeHS,HongJP.Diabetic foot reconstruction using free flaps increases 5-year-survival rate[J].J Plast Reconstr Aesthet Surg,2013,66(2):243-250.DOI: 10.1016/j.bjps.2012.09.024. [81] PatelSR. Local random flaps for the diabetic foot[J]. Clin Podiatr Med Surg, 2022, 39(2): 321-330. DOI: 10.1016/j.cpm.2021.11.004. [82] RamanujamCL,StutoAC,ZgonisT.Use of local intrinsic muscle flaps for diabetic foot and ankle reconstruction: a systematic review[J].J Wound Care,2018,27(Suppl 9):S22-28.DOI: 10.12968/jowc.2018.27.Sup9.S22. [83] WangN,YangBH,WangG,et al.A meta-analysis of the relationship between foot local characteristics and major lower extremity amputation in diabetic foot patients[J].J Cell Biochem,2019,120(6):9091-9096.DOI: 10.1002/jcb.28183. [84] Van DammeH,LimetR.Amputation in diabetic patients[J].Clin Podiatr Med Surg,2007,24(3):569-582, x.DOI: 10.1016/j.cpm.2007.03.007. -
表1 2009版牛津大学循证医学中心证据分级标准
表1. Oxford Centre for Evidence-Based Medicine: levels of evidence (2009)
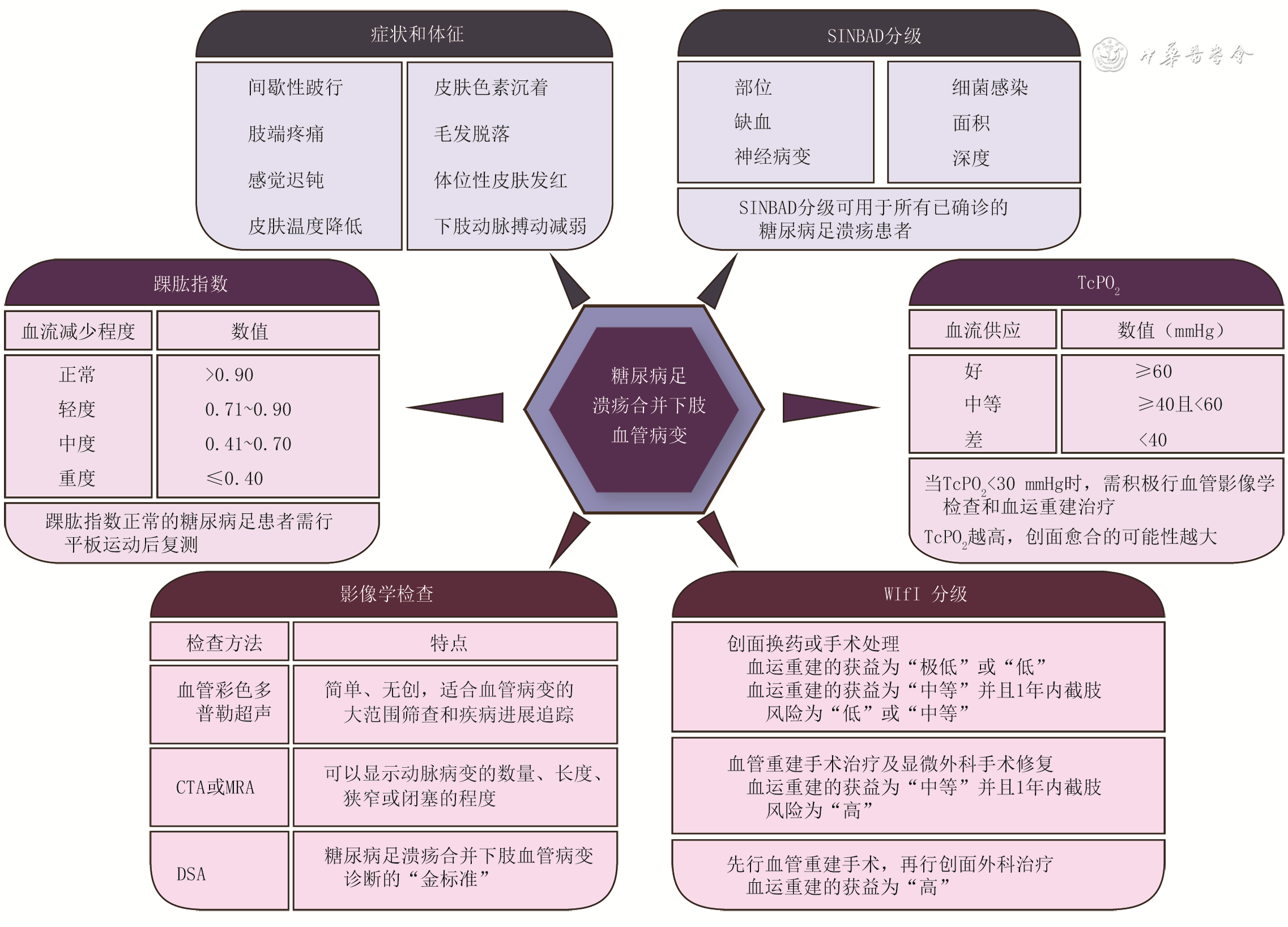
证据级别 定义 A 1a 基于同质RCT的系统评价 1b 单个RCT研究 1c 全或无病案研究 B 2a 基于同质队列研究的系统评价 2b 单个队列研究(包括低质量RCT,如随访率<80%) 2c 结果研究或生态学研究 3a 基于同质病例对照研究的系统评价 3b 病例对照研究 C 4 单个病例系列研究(包括低质量队列研究和病例对照研究) D 5 基于未经严格论证的专家意见 注:RCT为随机对照试验 表2 针对糖尿病足溃疡评估的SINBAD分级
表2. SINBAD classification for diabetic foot ulcer assessment
类型 定义 评分(分) 部位 前足掌 0 中、后足掌 1 缺血 足部血运正常,至少可触及1处动脉搏动 0 有足部血流减少的临床证据 1 神经病变 保护性感觉存在 0 保护性感觉缺失 1 细菌感染 无 0 有 1 面积 溃疡面积<1 cm 2 0 溃疡面积≥1 cm 2 1 深度 溃疡局限在皮肤和皮下组织 0 溃疡深达肌肉、肌腱或更深 1 注:表格引自文献[ 7];SINBAD为部位、缺血、神经病变、细菌感染、面积、深度 表3 针对糖尿病足溃疡患者的WIfI分级各组合的1年内截肢风险等级
表3. The risk grade of amputation within one year for each combination of WIfI classification for patients with diabetic foot ulcers
创面等级 0级缺血 1级缺血 2级缺血 3级缺血 0级足感染 1级足感染 2级足感染 3级足感染 0级足感染 1级足感染 2级足感染 3级足感染 0级足感染 1级足感染 2级足感染 3级足感染 0级足感染 1级足感染 2级足感染 3级足感染 0 极低 极低 低 中等 极低 低 中等 高 低 低 中等 高 低 中等 中等 高 1 极低 极低 低 中等 极低 低 中等 高 低 中等 高 高 中等 中等 高 高 2 低 低 中等 中等 中等 中等 高 高 中等 高 高 高 高 高 高 高 3 中等 中等 高 高 高 高 高 高 高 高 高 高 高 高 高 高 注:WIfI为创面、缺血和足感染 表4 针对糖尿病足溃疡患者的WIfI分级各组合的血运重建获益等级
表4. The benefit grade of revascularization for each combination of WIfI classification for patients with diabetic foot ulcers
创面等级 0级缺血 1级缺血 2级缺血 3级缺血 0级足感染 1级足感染 2级足感染 3级足感染 0级足感染 1级足感染 2级足感染 3级足感染 0级足感染 1级足感染 2级足感染 3级足感染 0级足感染 1级足感染 2级足感染 3级足感染 0 极低 极低 极低 极低 极低 低 低 中等 低 低 中等 中等 中等 高 高 高 1 极低 极低 极低 极低 低 中等 中等 高 中等 高 高 高 高 高 高 高 2 极低 极低 极低 极低 中等 中等 高 高 高 高 高 高 高 高 高 高 3 极低 极低 极低 极低 中等 中等 中等 高 高 高 高 高 高 高 高 高 注:WIfI为创面、缺血和足感染 -
 糖尿病足溃疡合并下肢血管病变的外科诊疗全国专家共识(2024版)-附件表格.docx
糖尿病足溃疡合并下肢血管病变的外科诊疗全国专家共识(2024版)-附件表格.docx

-








 下载:
下载: