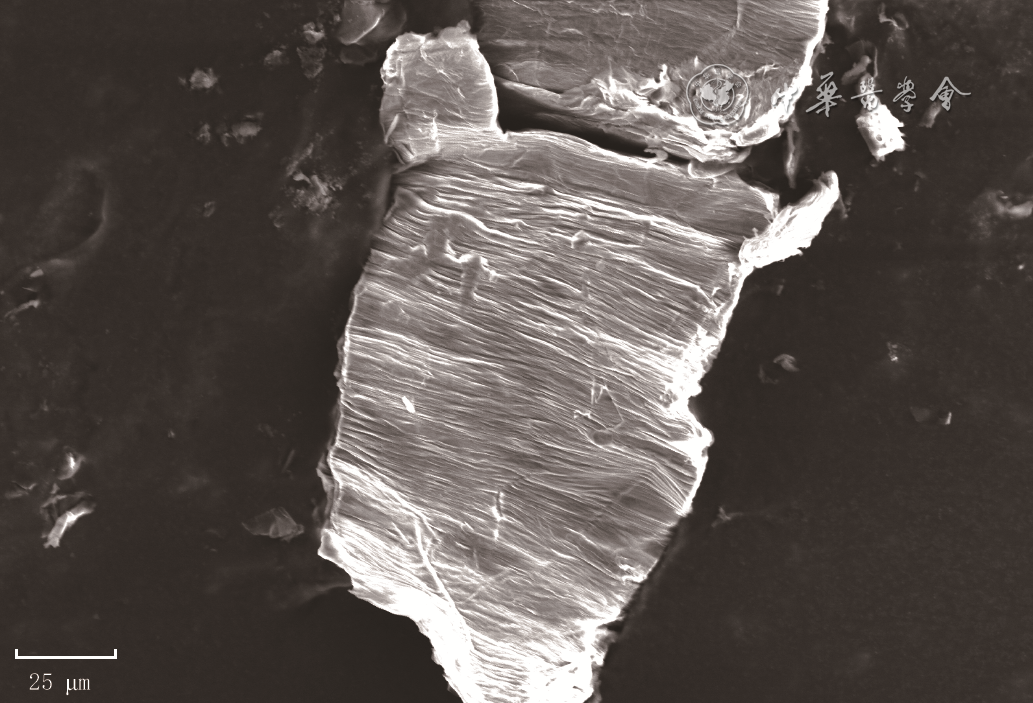
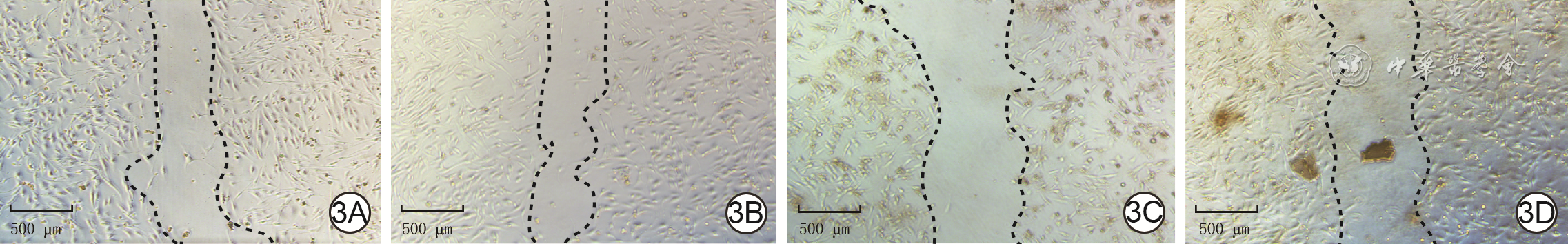
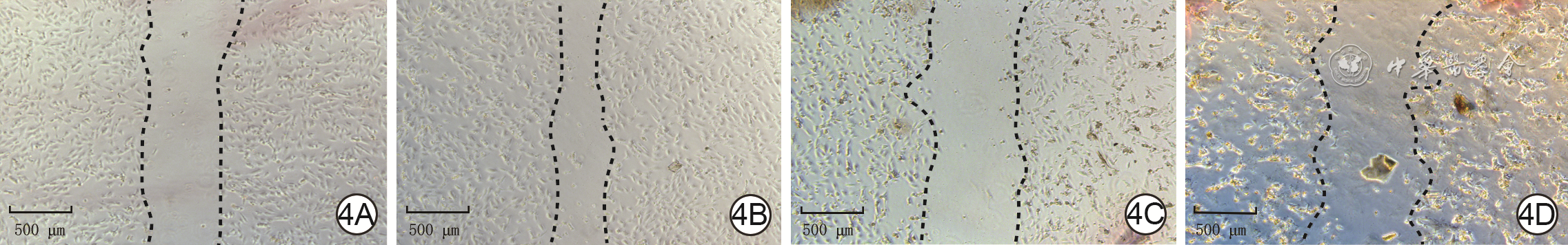
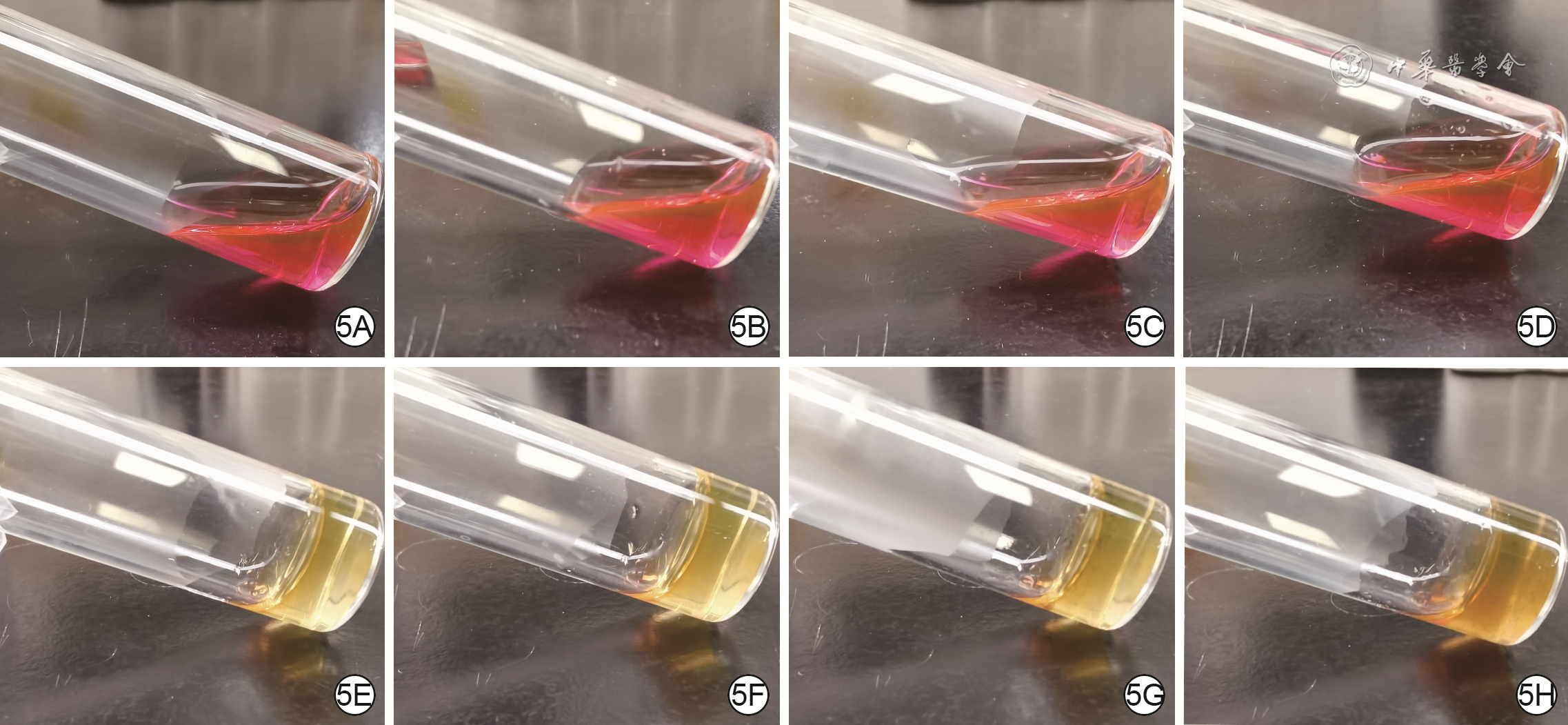
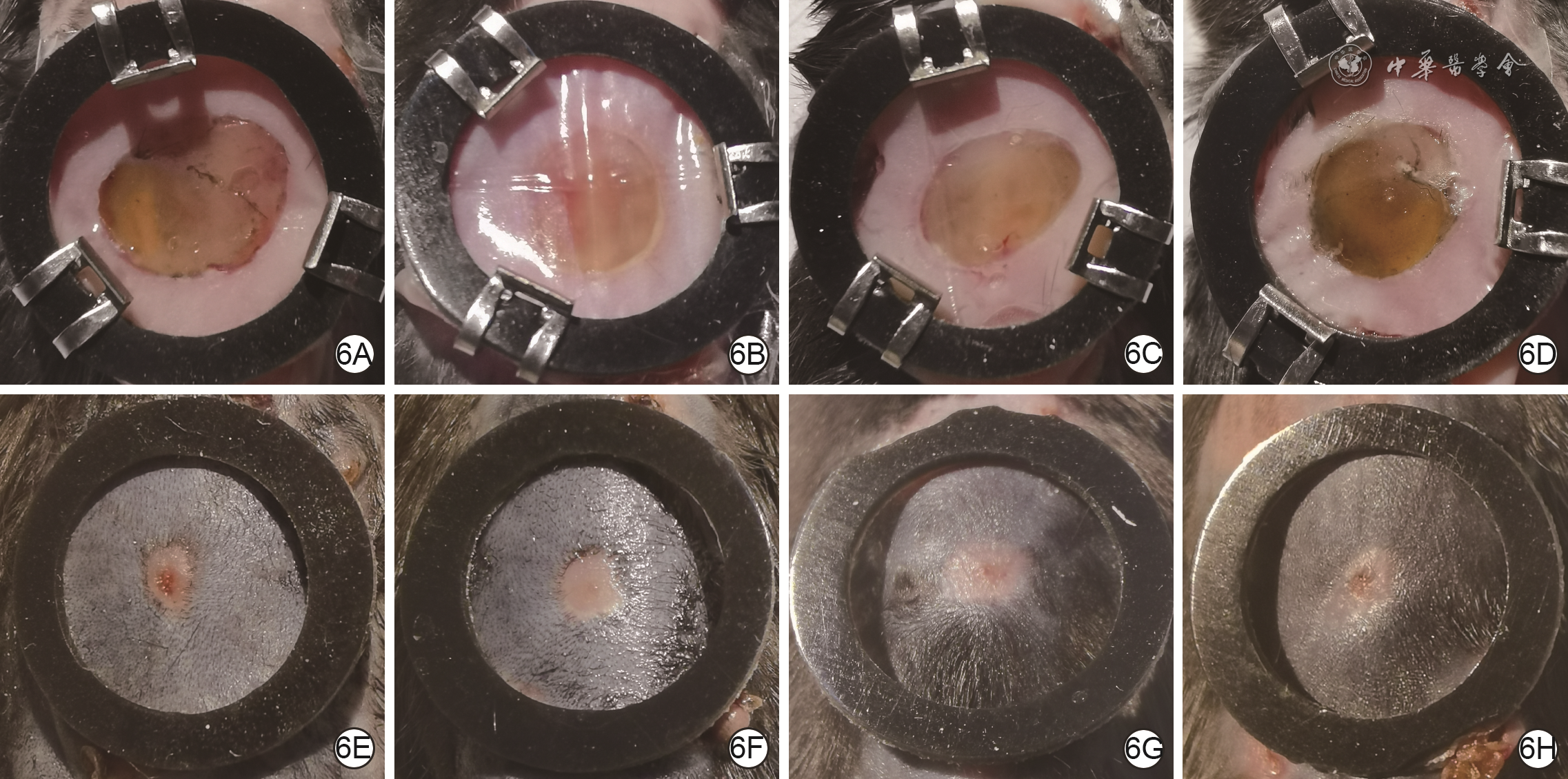
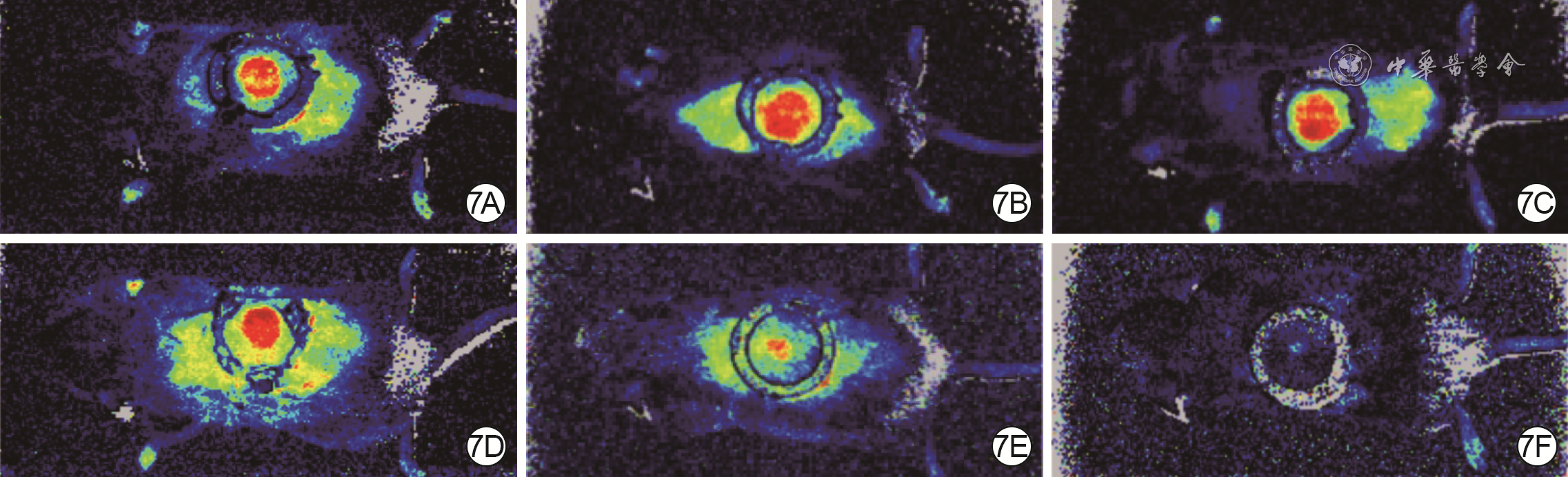
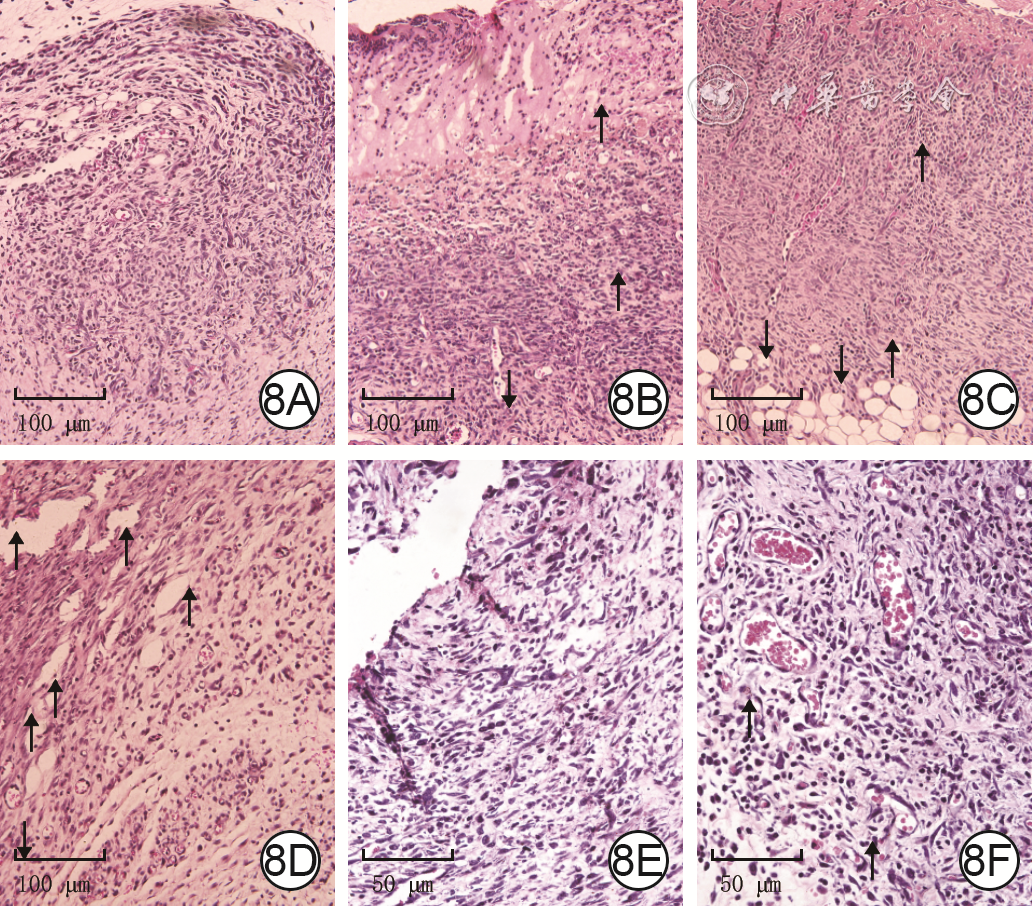
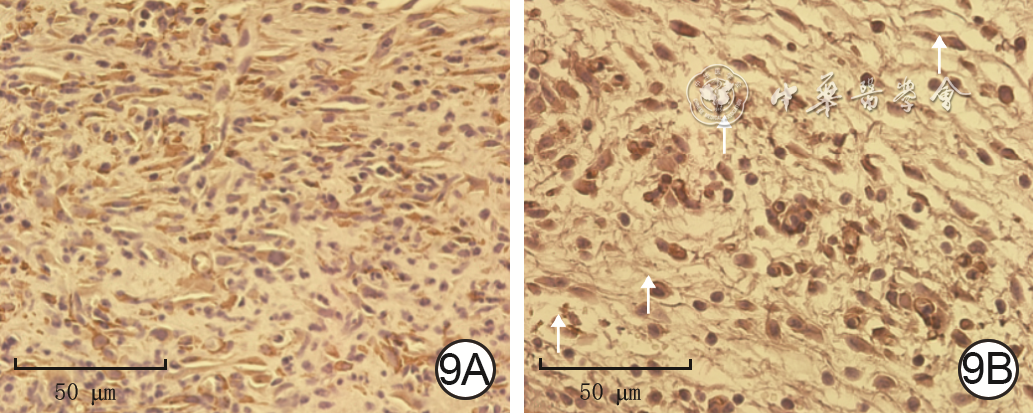
| [1] |
GuillemotF,SouquetA,CatrosS,et al.High-throughput laser printing of cells and biomaterials for tissue engineering[J].Acta Biomater,2010,6(7):2494-2500.DOI: 10.1016/j.actbio.2009.09.029. |
| [2] |
CampbellPG,WeissLE.Tissue engineering with the aid of inkjet printers[J].Expert Opin Biol Ther,2007,7(8):1123-1127.DOI: 10.1517/14712598.7.8.1123. |
| [3] |
OzbolatIT.Bioprinting scale-up tissue and organ constructs for transplantation[J].Trends Biotechnol,2015,33(7):395-400.DOI: 10.1016/j.tibtech.2015.04.005. |
| [4] |
LiX, LianQ, LiD, et al. Development of a robotic arm based hydrogel additive manufacturing system for in-situ printing[J]. Applied Sciences, 2017, 7(1):73. DOI: 10.3390/app7010073. |
| [5] |
SinghS,ChoudhuryD,YuF,et al.In situ bioprinting - bioprinting from benchside to bedside?[J].Acta Biomater,2020,101:14-25.DOI: 10.1016/j.actbio.2019.08.045. |
| [6] |
HuangR,HuJ,QianW,et al.Recent advances in nanotherapeutics for the treatment of burn wounds[J/OL].Burns Trauma,2021,9:tkab026[2022-03-14].https://pubmed.ncbi.nlm.nih.gov/34778468/.DOI: 10.1093/burnst/tkab026. |
| [7] |
WangH,LiuY,CaiK,et al.Antibacterial polysaccharide-based hydrogel dressing containing plant essential oil for burn wound healing[J/OL].Burns Trauma,2021,9:tkab041[2022-03-14].https://pubmed.ncbi.nlm.nih.gov/34988231/.DOI: 10.1093/burnst/tkab041. |
| [8] |
AfewerkiS,SheikhiA,KannanS,et al.Gelatin-polysaccharide composite scaffolds for 3D cell culture and tissue engineering: towards natural therapeutics[J].Bioeng Transl Med,2018,4(1):96-115.DOI: 10.1002/btm2.10124. |
| [9] |
LiMN,YuHP,KeQF,et al.Gelatin methacryloyl hydrogels functionalized with endothelin-1 for angiogenesis and full- thickness wound healing[J].J Mater Chem B,2021,9(23):4700-4709.DOI: 10.1039/d1tb00449b. |
| [10] |
VandoorenJ,Van den SteenPE,OpdenakkerG.Biochemistry and molecular biology of gelatinase B or matrix metalloproteinase-9 (MMP-9): the next decade[J].Crit Rev Biochem Mol Biol,2013,48(3):222-272.DOI: 10.3109/10409238.2013.770819. |
| [11] |
JangMJ,BaeSK,JungYS,et al.Enhanced wound healing using a 3D printed VEGF-mimicking peptide incorporated hydrogel patch in a pig model[J].Biomed Mater,2021,16(4):605.DOI: 10.1088/1748-605X/abf1a8. |
| [12] |
LoessnerD,MeinertC,KaemmererE,et al.Functionalization, preparation and use of cell-laden gelatin methacryloyl-based hydrogels as modular tissue culture platforms[J].Nat Protoc,2016,11(4):727-746.DOI: 10.1038/nprot.2016.037. |
| [13] |
KlotzBJ,GawlittaD,AJWPRosenberg,et al.Gelatin-methacryloyl hydrogels: towards biofabrication-based tissue repair[J].Trends Biotechnol,2016,34(5):394-407.DOI: 10.1016/j.tibtech.2016.01.002. |
| [14] |
NuutilaK,SamandariM,EndoY,et al.In vivo printing of growth factor-eluting adhesive scaffolds improves wound healing[J].Bioact Mater,2022,8:296-308.DOI: 10.1016/j.bioactmat.2021.06.030. |
| [15] |
ZhouX,ChenJ,SunH,et al.Spatiotemporal regulation of angiogenesis/osteogenesis emulating natural bone healing cascade for vascularized bone formation[J].J Nanobiotechnology,2021,19(1):420.DOI: 10.1186/s12951-021-01173-z. |
| [16] |
ChenYC,LinRZ,QiH,et al.Functional human vascular network generated in photocrosslinkable gelatin methacrylate hydrogels[J].Adv Funct Mater,2012,22(10):2027-2039.DOI: 10.1002/adfm.201101662. |
| [17] |
LinRZ,ChenYC,Moreno-LunaR,et al.Transdermal regulation of vascular network bioengineering using a photopolymerizable methacrylated gelatin hydrogel[J].Biomaterials,2013,34(28):6785-6796.DOI: 10.1016/j.biomaterials.2013.05.060. |
| [18] |
FangH,LuoC,LiuS,et al.A biocompatible vascularized graphene oxide (GO)-collagen chamber with osteoinductive and anti- fibrosis effects promotes bone regeneration in vivo[J].Theranostics,2020,10(6):2759-2772.DOI: 10.7150/thno.42006. |
| [19] |
NorahanMH,AmroonM,GhahremanzadehR,et al.Electroactive graphene oxide-incorporated collagen assisting vascularization for cardiac tissue engineering[J].J Biomed Mater Res A,2019,107(1):204-219.DOI: 10.1002/jbm.a.36555. |
| [20] |
ChakrabortyS,PonrasuT,ChandelS,et al.Reduced graphene oxide-loaded nanocomposite scaffolds for enhancing angiogenesis in tissue engineering applications[J].R Soc Open Sci,2018,5(5):172017.DOI: 10.1098/rsos.172017. |
| [21] |
MukherjeeS,SriramP,BaruiAK,et al.Graphene oxides show angiogenic properties[J].Adv Healthc Mater,2015,4(11):1722-1732.DOI: 10.1002/adhm.201500155. |
| [22] |
ChangTK,LuYC,YehST,et al.In vitro and in vivo biological responses to graphene and graphene oxide: a murine calvarial animal study[J].Int J Nanomedicine,2020,15:647-659.DOI: 10.2147/IJN.S231885. |
| [23] |
LiuW,LuoH,WeiQ,et al.Electrochemically derived nanographene oxide activates endothelial tip cells and promotes angiogenesis by binding endogenous lysophosphatidic acid[J].Bioact Mater,2022,9:92-104.DOI: 10.1016/j.bioactmat.2021.07.007. |
| [24] |
焦德龙功能化石墨烯/光凝胶复合载药体系用于骨组织再生修复研究上海上海交通大学2019DOI:10.27307/d.cnki.gsjtu.2019.004439焦德龙.功能化石墨烯/光凝胶复合载药体系用于骨组织再生修复研究[D].上海:上海交通大学,2019.DOI:10.27307/d.cnki.gsjtu.2019.004439.
|
| [25] |
YueK,Trujillo-de SantiagoG,AlvarezMM,et al.Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels[J].Biomaterials,2015,73:254-271.DOI: 10.1016/j.biomaterials.2015.08.045. |
| [26] |
CoentroJQ,PuglieseE,HanleyG,et al.Current and upcoming therapies to modulate skin scarring and fibrosis[J].Adv Drug Deliv Rev,2019,146:37-59.DOI: 10.1016/j.addr.2018.08.009. |
| [27] |
VedakumariSW,Veda JancySJ,PravinYR,et al.Facile synthesis of sericin modified graphene oxide nanocomposites for treating ischemic diseases[J].Environ Res,2022,209:112925.DOI: 10.1016/j.envres.2022.112925. |
| [28] |
VerdeV,LongoA,CucciLM,et al.Anti-angiogenic and anti-proliferative graphene oxide nanosheets for tumor cell therapy[J].Int J Mol Sci,2020,21(15):5571.DOI: 10.3390/ijms21155571. |
| [29] |
LaiPX,ChenCW,WeiSC,et al.Ultrastrong trapping of VEGF by graphene oxide: anti-angiogenesis application[J].Biomaterials,2016,109:12-22.DOI: 10.1016/j.biomaterials.2016.09.005. |
| [30] |
ZhangY,AliSF,DervishiE,et al.Cytotoxicity effects of graphene and single-wall carbon nanotubes in neural phaeochromocytoma-derived PC12 cells[J].ACS Nano,2010,4(6):3181-3186.DOI: 10.1021/nn1007176. |
| [31] |
LiangY,ChenB,LiM,et al.Injectable antimicrobial conductive hydrogels for wound disinfection and infectious wound healing[J].Biomacromolecules,2020,21(5):1841-1852.DOI: 10.1021/acs.biomac.9b01732. |
| [32] |
GurtnerGC,WernerS,BarrandonY,et al.Wound repair and regeneration[J].Nature,2008,453(7193):314-321.DOI: 10.1038/nature07039. |
| [33] |
LiM,ZhaoY,HaoH,et al.Theoretical and practical aspects of using fetal fibroblasts for skin regeneration[J].Ageing Res Rev,2017,36:32-41.DOI: 10.1016/j.arr.2017.02.005. |
| [34] |
WangM,WangC,ChenM,et al.Efficient angiogenesis-based diabetic wound healing/skin reconstruction through bioactive antibacterial adhesive ultraviolet shielding nanodressing with exosome release[J].ACS Nano,2019,13(9):10279-10293.DOI: 10.1021/acsnano.9b03656. |
| [35] |
RidiandriesA,TanJ,BursillCA.The role of chemokines in wound healing[J].Int JTM Mol Sci,2018,19(10):3217.DOI: 10.3390/ijms19103217. |
| [36] |
|
| [37] |
AllenRG,BalinAK.Oxidative influence on development and differentiation: an overview of a free radical theory of development[J].Free Radic Biol Med,1989,6(6):631-661.DOI: 10.1016/0891-5849(89)90071-3. |
| [38] |
CalabreseV,MancusoC,CalvaniM,et al.Nitric oxide in the central nervous system: neuroprotection versus neurotoxicity[J].Nat Rev Neurosci,2007,8(10):766-775.DOI: 10.1038/nrn2214. |
| [39] |
|
| [40] |
ChongY,GeC,YangZ,et al.Reduced cytotoxicity of graphene nanosheets mediated by blood-protein coating[J].ACS Nano,2015,9(6):5713-5724.DOI: 10.1021/nn5066606. |
| [41] |
WeiXQ,HaoLY,ShaoXR,et al.Insight into the interaction of graphene oxide with serum proteins and the impact of the degree of reduction and concentration[J].ACS Appl Mater Interfaces,2015,7(24):13367-13374.DOI: 10.1021/acsami.5b01874. |
| [42] |
FengR,YuY,ShenC,et al.Impact of graphene oxide on the structure and function of important multiple blood components by a dose-dependent pattern[J].J Biomed Mater Res A,2015,103(6):2006-2014.DOI: 10.1002/jbm.a.35341. |
| [43] |
LiS,MulloorJJ,WangL,et al.Strong and selective adsorption of lysozyme on graphene oxide[J].ACS Appl Mater Interfaces,2014,6(8):5704-5712.DOI: 10.1021/am500254e. |







 下载:
下载:





 DownLoad:
DownLoad: